Our Science
Unlocking a new era of medical innovation
ImmuPharma Biotech excels in harnessing the transformative potential of peptides, driving the future of programmable therapies with cutting-edge research.
Therapeutic Repositioning Platform for Autoimmune Diseases

Unique mechanism of action
Evidence suggests that P140 restores the immune tolerance systems by enabling tolerogenic antigen presenting cells (like dendritic cells) to function properly. As malfunction of the tolerance systems seems to be the root cause of most, if not all autoimmune diseases. This explains why P140 is so broadly efficient across most autoimmune indications in animal models. P140 is the only non-immunosuppressive molecule in the industry, in clinical development, for the treatment of SLE. All other attempts to treat autoimmunity involve suppressing the immune system.
This “restoration” of the immune system marks a paradigm shift in treating autoimmune disease, and explains why P140 is not an immunosuppressant.
Target diseases
Near term: SLE • CIDP
Long term: Periodontitis • Asthma • Gout • IBD
ADMET Programming Technology
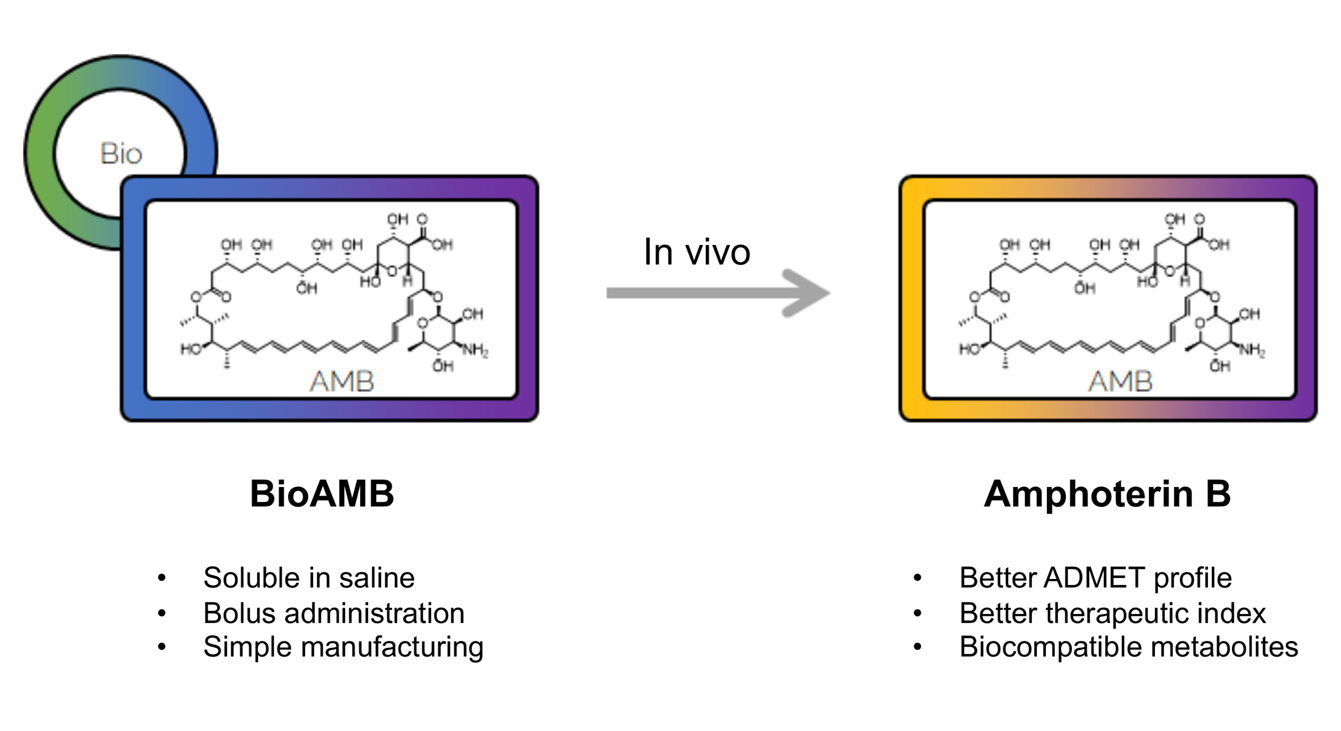
Taking old drugs and adapting them to develop new and more effective drugs
BioAMB and BioCIN are two advanced preclinical research projects that stand to strategically pivot the market positioning of these assets towards first-line therapy options.
Much like peptides, we’ve recognised that a multitude of products have their utility capped due to bioavailability hurdles. This issue is strikingly prevalent in anti-infective compounds. Increased antibiotic and anti-fungal resistance is one of the biggest threats to global health, cost and mortality. Greater awareness from the World Health Organisation and US CDC is beginning to draw attention to the growing need for more anti-infective drugs to battle life-threatening conditions, particularly in the face of increasing resistance by organisms to existing drugs. As an example, significant problems can occur in immunosuppressed patients which may lead to serious fungal infections.
Stemming from these insights, two distinct programs have emerged within our R&D unit, each aiming to enhance patient safety with two strategic drugs: Amphotericin-B for fungal lung infections and Vancomycin for gram-negative bacterial lung infections. Despite their potency against respective pathogens, both compounds present administration and toxicity challenges, restricting them to be used only as last-resort treatments. Our cutting-edge ADMET property programming technology has led to a significant reduction in administration times and has simplified the formulation for each product. Moreover, a series of in-vivo experiments have bolstered our confidence regarding the decrease in associated toxicity.
Target diseases
Chronic Pulmonary Aspergillosis • Invasive Aspergillosis • Skin & Bone infections • MRSA